念書筆記
Ref: Epigenetic clocks, aging, and cancer
Epigenetic clocks, aging, and cancer | Science
DOI: 10.1126/science.abn4009
摘要
Methylation is copied during DNA replication by DNA methyltransferases (DNMTs).
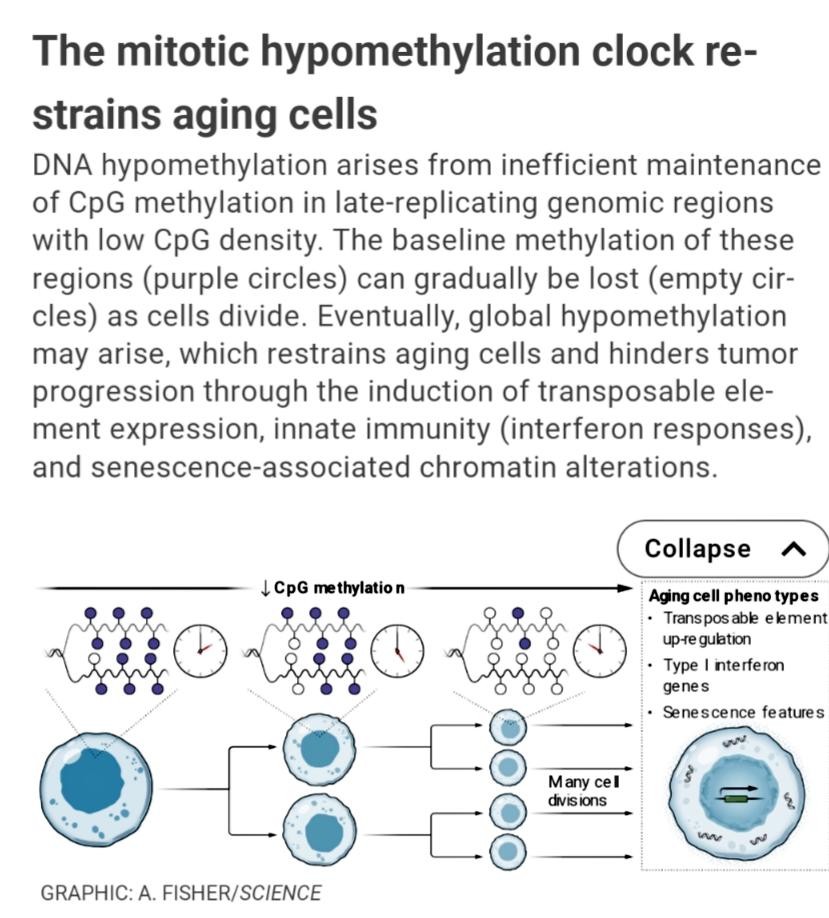
In tumors, hypomethylation affects megabase-sized intervals that are CpG-poor, gene-poor, and late-replicating. Tissues from older individuals also exhibit widespread hypomethylation of the same regions as in cancer, albeit of lesser magnitude. Hypomethylation is more pronounced in proliferative epithelial tissues, compared with brain tissue and other compartments that largely comprise postmitotic cells. Moreover, primary cells become progressively hypomethylated with increasing passages in culture, but their methylation stabilizes when they exit the cell cycle (5). In cancer cells, the degree of hypomethylation correlates with somatic mutational burden, which is established to have mitotic clock**–**like properties because the number of mutations increases as cells accumulate divisions (6).
- 失去methylation與老化,癌化有關,尤其在gene-poor region這問題更顯嚴重。
Substantial evidence supports several such restrictive functions for hypomethylation. DNA methylation plays a fundamental role in the transcriptional silencing of transposable elements (TEs) such as endogenous retroviruses (ERVs) and long interspersed nuclear elements (LINEs), which are inserted at high copy numbers throughout mammalian genomes (12). The expression of TEs is strongly up-regulated across a range of tumors, particularly in hypomethylated regions. In support of a causal role for methylation loss, the DNMT inhibitor 5-azacytidine induces LINE and ERV expression in vitro and in vivo (13).
TE reactivation triggers innate immunity through viral mimicry (13). The derepressed elements produce double-stranded RNAs, which are sensed by cytosolic sensors that trigger a type I interferon (IFN) response. DNMT inhibitors activate TEs in cancer cells, increase immune infiltration, and sensitize tumors to immunotherapy. LINE reactivation has also been documented in senescent cells, where it triggers IFN responses and may promote inflammaging, a chronic inflammatory state linked to aging-associated diseases (12).
- 而且失去methylation會讓TE活化,尤其像癌細胞就是一團TE活化的結果
- 而且TE活化也會產生double strand RNA進而活化innate immunity,開啟type I interferon
- LINE活化也常見於老化細胞
Although hypomethylation is conserved across mammals, its pace varies between organisms and cell types. Accelerated global hypomethylation was proposed to explain the rarity of tumors in blind mole rats, a species notable for longevity and cancer resistance (15). So although hypomethylation may reflect an inherent feature of mammalian genomes, it may be “tunable” across species and tissues. This is reminiscent of the heterochromatin-based mitotic clock in the yeast Saccharomyces cerevisiae, whose modulation alters life span (5). There are also parallels to somatic mutational rates, whose variability across mammals scales with life span (6). Moreover, the extent to which hypomethylation primes downstream expression changes and phenotypes may represent an additional tunable component of the mitotic clock. Even to the extent that hypomethylation is an intrinsic feature of mammalian genomes predicated in imperfect maintenance, its pace and downstream integration by chromatin and transcriptional machinery have likely evolved to balance cancer resistance and aging phenotypes.
- 雖然hypomethylation可能是哺乳類生物共有的現象,但依舊存在tissue slecific and cell type specific pattern,而且這件事情可能是tunable的
| ref: https://www.science.org/doi/10.1126/science.abn4009#f1 |
|---|
 |
Take home messages
老化、癌化與hypomethylation有關,而hypomethylation之後會讓TE過度活化,造成後續一系列的問題。雖有cell type-specific and species-specific,但這套機制應該是conserved
Keywords
mitotic hypomethylation, mitotic clock, TE, methylation, epigenic